Introduction
During the Covid-19 pandemic, many laboratories focused their attention on antiviral research. Working with this highly infectious virus requires access to biosafety level 3 labs. However, there is a shortage of this type of lab worldwide, so the bacteriophage phi6 is used as a non-pathogenic surrogate. This is largely based on the fact that phi6 is a lipid enveloped RNA virus and its stability in the environment enables a legitimate comparison to the behaviors of the other enveloped RNA viruses such as SARS-CoV-2, Ebola virus or rotavirus [1] [2].
Such virus research and various other virus-based applications require highly purified viruses or virus-like particles (VLPs). Viruses can be propagated to large quantities in bioreactors. Due to the need for living cells for propagation the starting material for virus purification is biologically complex and thus quite challenging [3].
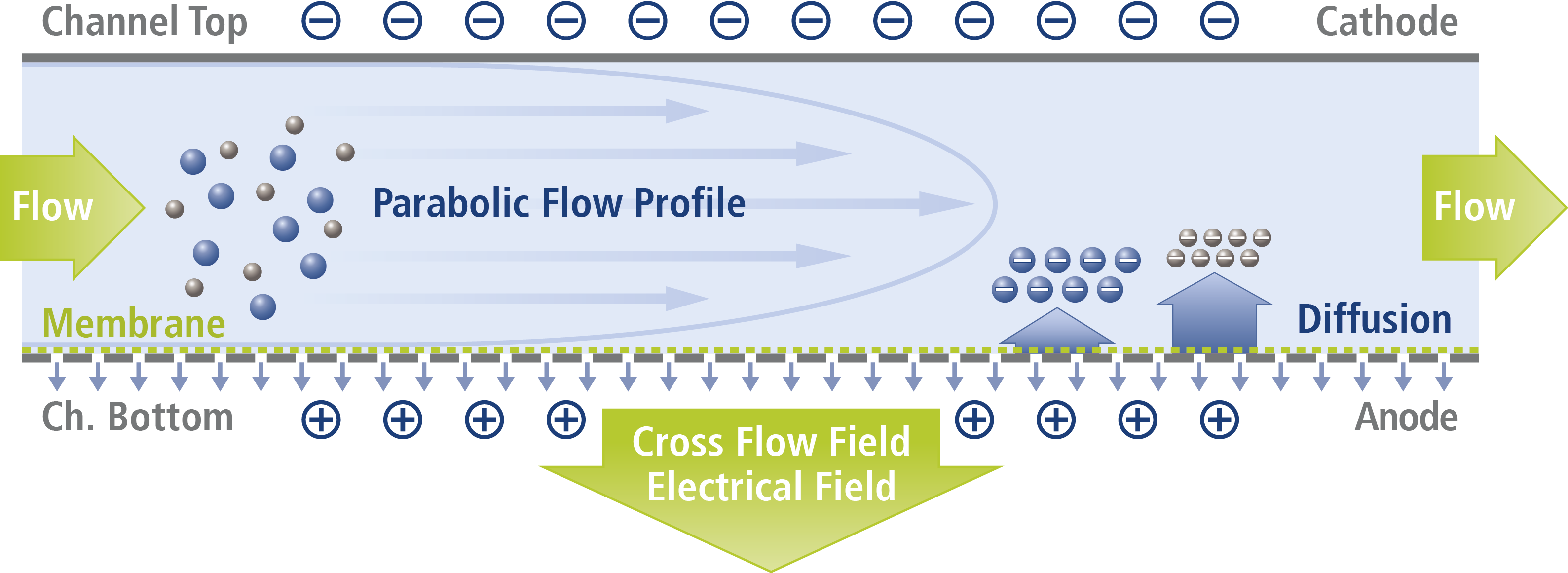
Asymmetrical Flow Field-Flow Fractionation (AF4) is a promising method for virus purification [3] also enabling process monitoring due to a comprehensive characterization of the viruses or VLPs. Here we demonstrate the measurement of the phi6 virus and its characterization according to size and charge by Electrical Asymmetrical Field-Flow Fractionation (EAF4). A schematic for the EAF4 channel and its separation principle is shown in the appendix.
Experimental
The phi6 sample (concentration 14 mg mL-1) was diluted 1:50 in a solvent of 20 mM phosphate buffer (potassium salts) and 1 mM magnesium chloride (pH = 7.2), which was also used as eluent for the EAF4 measurements. The mixture was analyzed by EAF4 using four different electrical field conditions enabling measurement of electrophoretic mobility and thus the determination of the surface zeta potential. In addition, Multi-Angle Light Scattering (MALS) and Dynamic Light Scattering (DLS) were used as detectors to simultaneously collect information about size (Radius of gyration Rg in case of MALS and hydrodynamic radius Rh in case of DLS).
Results
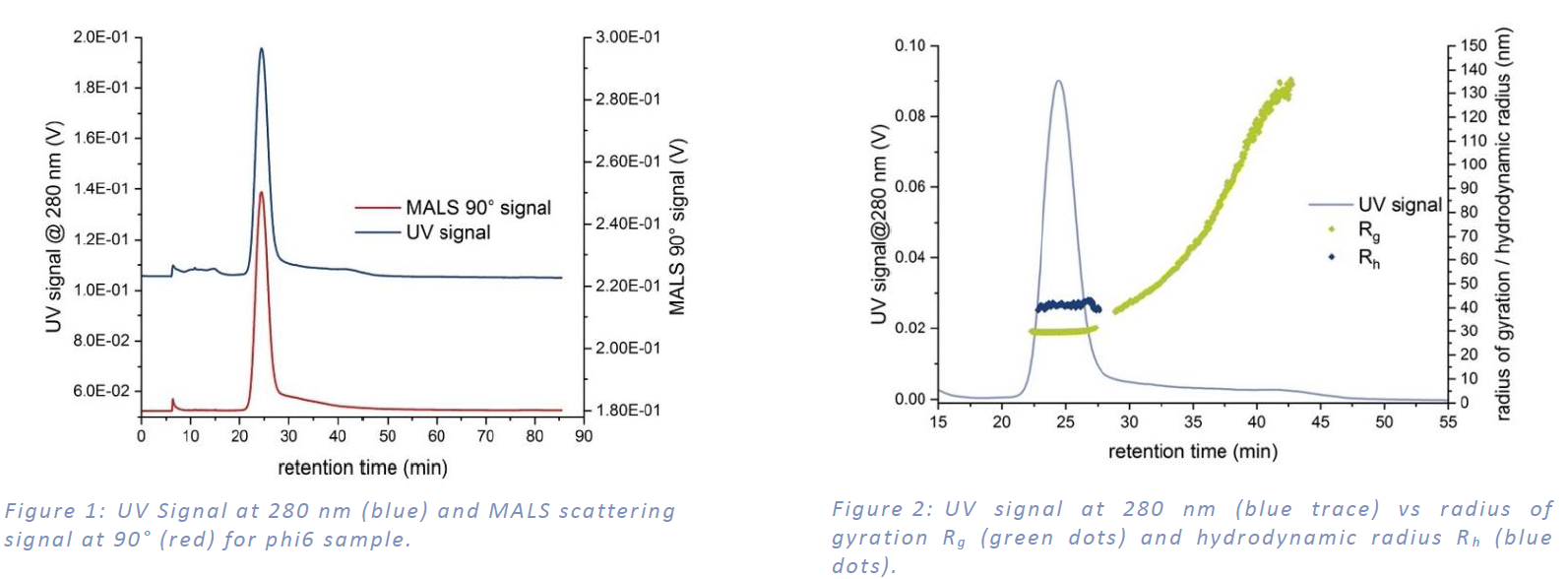
MALS and UV detection show the main signal peak between 20 min and 30 min and a second fraction up to 50 min with a recovery of more than 90% (see Figure 1:). Rg was calculated from MALS angular data and Rh was determined by cumulants analysis from online DLS measurements. Figure 2: shows both radii. In the main peak the Rg is 29.1 nm ± 0.4 nm and the Rh is 40.5 nm ± 1.2 nm. The ratio of Rg to Rh of 0.72 indicates a spherical shape. Larger sample constituents showed an increasing Rg up to around 120 nm.
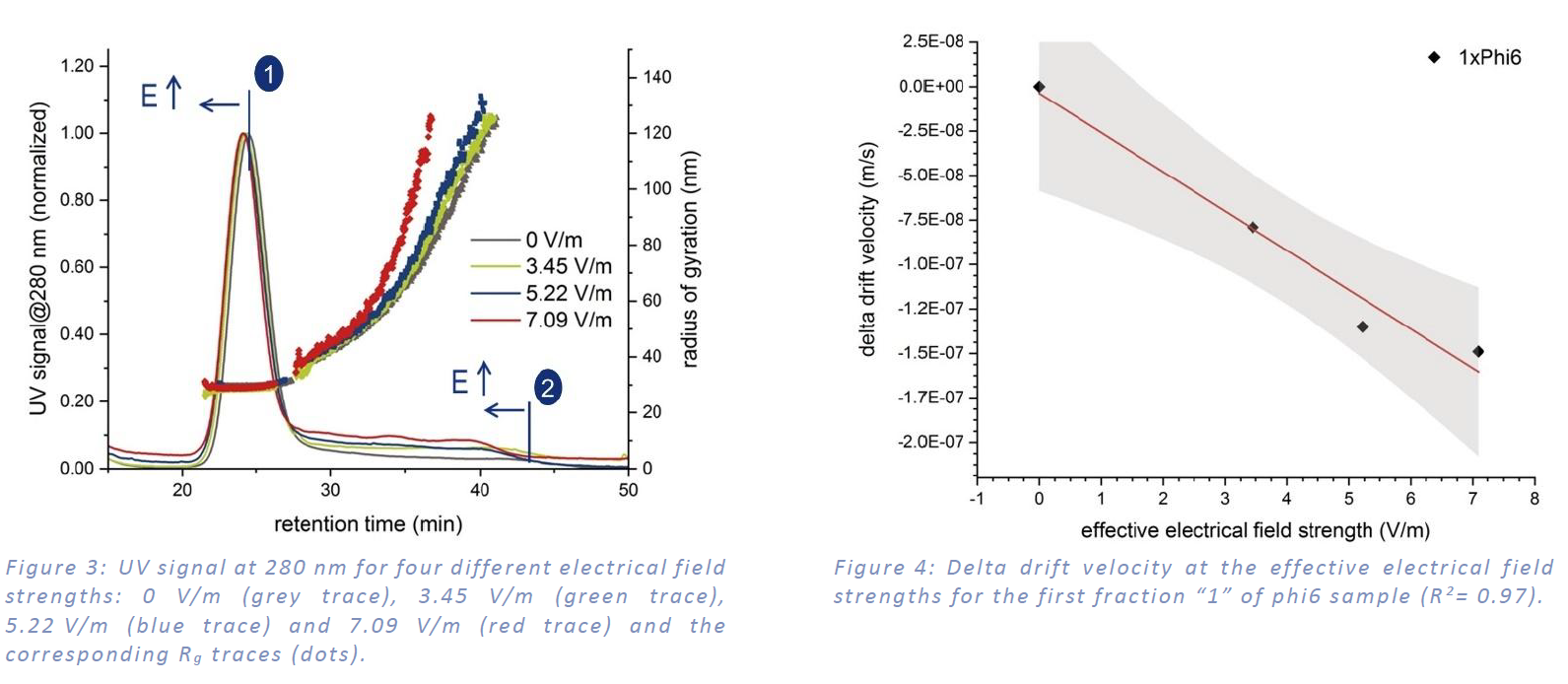
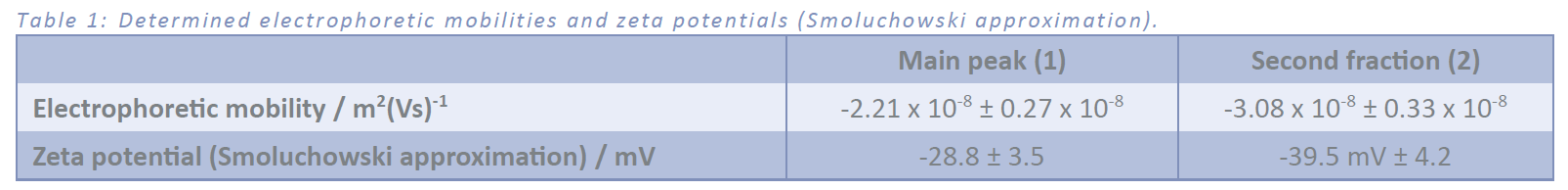
The electrophoretic mobility was determined from four measurements using different electrical field strengths. The bottom plate was charged negatively. Figure 3: shows the decreasing retention time of both peaks with increasing field strength due to the negative surface charge of the sample. The calculation of electrophoretic mobility is displayed in Figure 4 and Table 1 summarizes the results. The size distribution doesn´t change due to the application of an electrical field. Since the second fraction “2” isn’t a fully resolved peak, the determination of the electrophoretic mobility is more difficult and contains a higher error. The higher electrophoretic mobility for the second fraction indicates a slightly higher surface charge density compared to the first fraction.
Conclusion
Asymmetrical Flow Field-Flow Fractionation coupled with UV-, MALS- and DLS-detectors is a powerful tool for separation of a phi6 virus sample and its characterization according to size and shape within one single run. Adding an electrical field on top of the crossflow field allows for the additional determination of the size-resolved electrophoretic mobility and the surface Zeta potential of the single fractions.
References
[1] A. Serrano-Aroca, “Antiviral Characterization of Advanced Materials: Use of Bacteriophage Phi 6 as Surrogate of Enveloped Viruses Such as SARS-CoV-2” International Journal of Molecular Sciences, 2022, 23, 5335.
[2] C. Whitworth, Y. Mu, H. Houston, M. Martinez-Smith, J. Noble-Wang, A. Coulliette-Salmond und L. Rose, “Persistence of Bacteriophage Phi 6 on Porous and Nonporous Surfaces and the Potential for Its Use as an Ebola Virus or Coronavirus Surrogate”, Applied and Environmental Microbiology, 2020, 86(17), 1482-20.
[3] M. Lampi, H. M. Oksanen, F. Meier, E. Moldenhauer, M. M. Poranen, D. H. Bamford und K. Eskelin, “Asymmetric flow field flow fractionation methods for virus purification”, Journal of Chromatography B, 2018, 1095, 251-257.
Appendix
Schematic representation of an EAF4 channel used for the analysis of negatively charged particles where the accumulation wall (membrane) was chosen to be the anode. The polarity of the channel walls can be changed readily according to the particle charge type and desired direction of the electrical field.