Introduction
Adeno-associated viruses (AAVs) have emerged as promising vectors for gene therapy due to their low immunogenicity and ability to transduce a variety of cell types [1]. However, the development of safe and effective AAV-based therapeutics necessitates accurate and comprehensive characterization of these vectors. Key challenges in AAV analysis include determining particle size distribution, distinguishing between full and empty capsids, accurately measuring vector titer, and identifying protein impurities [1] [2]. Traditional methods often fall short in providing the required resolution and accuracy [3]. This application note presents an advanced analytical approach using Multi-Detector Asymmetrical Flow Field-Flow Fractionation (MD-AF4) to address these challenges. MD-AF4 offers a gentle, high-resolution, label-free technique for detailed evaluation of AAV8 vectors. The findings demonstrate the effectiveness of MD-AF4 in providing robust, reliable results that align with expectations and manufacturing requirements, offering a powerful tool for AAV characterization in gene therapy research and development.
Experimental
The AAV8 sample was generated, purified, and provided by Sartorius BIA Separations. The AAV8 sample was purified using cation-exchange (CEX) chromatography on a strong CEX monolithic column – CIMmultus SO3 1 mL preparative column with 2 μm channels (Sartorius BIA Separations). The AAV8 sample with encapsidated ssDNA cargo was analyzed using Postnova's AF2000 Multi Flow FFF system, equipped with UV-DAD, multi-angle light scattering (MALS), refractive index (RI), and dynamic light scattering (DLS) detectors. The analysis was performed with 1× PBS (pH 7.4) as the carrier solution. The sample stream was concentrated fourfold using the Smart-Stream-Splitting module. A 20 μL aliquot of the AAV sample was injected into a standard AF4 channel containing a 10 kDa NovaRC membrane and a 350 μm spacer. Table 1 lists the sample properties, including the nominal molar masses of the empty capsid and ssDNA payload. The values for refractive index increments (dn/dc) and extinction coefficients were used for the compositional analysis, which was performed using NovaAnalysis Software. The ratio of full to total AAV8 capsids (vg/cp) was additionally determined by anion-exchange (AEX) chromatography, using a strong AEX monolithic column – CIMac QA HR 0.1 mL analytical column with 2 μm channels (Sartorius BIA Separations).
Result and Discussion
Particle size and molar mass distribution
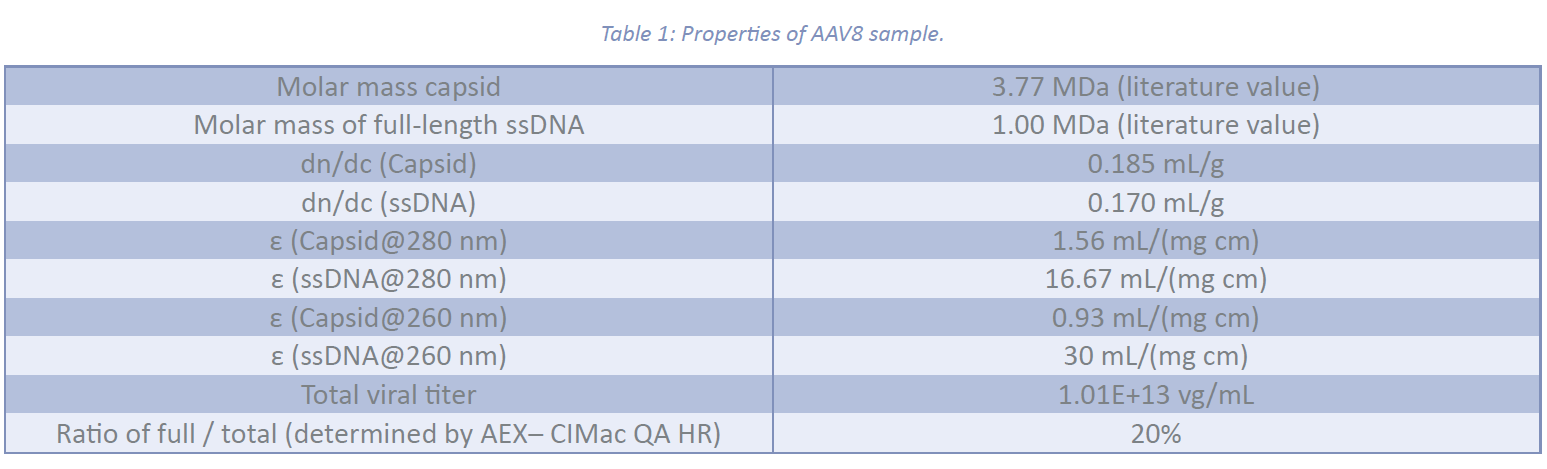
The profiles of the gyration radius (Rg) and hydrodynamic radius (Rh) as a function of elution time across the AAV8 fractogram are shown in Figure 1:. The Rh profile follows a zero-slope straight line, demonstrating a monodisperse hydrodynamic size distribution with a weight average radius of 13.8 ± 1.0 nm. However, the Rg profile changes slightly, ranging from 11 nm to 14 nm across the peak. This variation may be attributed to the presence of both empty and full capsids within the distribution. The weight average Rg was calculated to be 12.0 ± 0.1 nm. No significant fraction of agglomerates and impurities was identified.
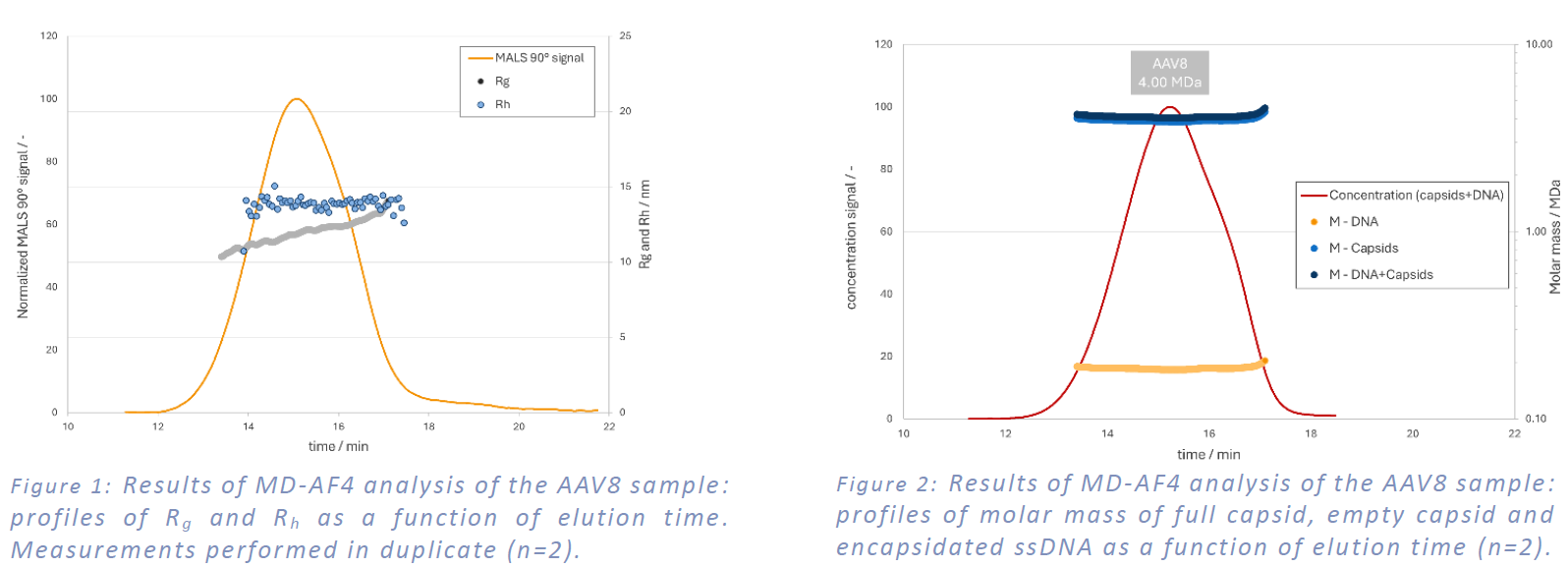
The molar mass profiles of the empty capsid, full capsid, and encapsidated ssDNA cargo, determined by compositional analysis, are presented in Figure 2:. This analysis utilized the optical properties of the AAV8 sample detailed in Table 1. The weight average total molar mass of the AAV8 (comprising the empty capsid and ssDNA) was measured to be 4.00 ± 0.1 MDa. The molar masses of the encapsidated ssDNA and empty capsid were found to be 0.182 ± 0.005 MDa and 3.80 ± 0.1 MDa, respectively. The measured molar masses of the encapsidated ssDNA cargo and total AAV8 were lower than their nominal values, suggesting that only a proportion of the capsids contained the ssDNA cargo (approximately 1 to 5).
Full to empty ratio and titer analysis
Compositional analysis also allows for the calculation of the number-based concentration (titer) of total and full capsids, using the eluted mass of ssDNA and capsid measured from concentration trace data. The analysis determined the titer of the total capsid to be 5.06E+13 cp/mL and the full capsid titer to be 9.04E+12 vg/mL, resulting in an encapsidation content of 17.9%. The measured values show good agreement with the vg/cp values obtained from orthogonal AEX analysis (20%) and total viral titer (1.01E+13 vg/mL).
Conclusion
MD-AF4 proves to be a powerful tool for the comprehensive characterization of AAV8 vectors. The system provides accurate and reliable measurements of particle size, molar mass, and full-to-empty capsid ratios. These results align with expected values and demonstrate the potential of MD-AF4 for AAV analysis, crucial for gene therapy development and quality control.
References
[1] T. Kontogiannis, J. Braybrook, C. McElroy, C. Foy, A. S. Whale, M. Quaglia, C. M. Smales, „Characterization of AAV vectors: A review of analytical techniques and critical quality attributes“, Molecular Therapy Methods & Clinical Development, 2024, 32(3), 101309.
[2] N. L. McIntosh, G. Y. Berguig, O. A. Karim, C. L. Cortesio, R. De Angelis, A. A. Khan, D. Gold, J. A. Maga, V. S. Bhat, „Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering“, Scientific Reports, 2021, 11(1), 3012.
[3] A. K. Werle, T. W. Powers, J. F. Zobel, C. N. Wappelhorst, M. F. Jarrold, N. A. Lyktey, C. D. K. Sloan, A. J. Wolf, S. Adams-Hall, P. Baldus, H. A. Runnels, „Comparison of analytical techniques