Introduction
Messenger RNA (mRNA) encapsulated in lipid nanoparticles (LNPs) has emerged as a transformative platform for therapeutics and vaccines, offering a highly adaptable and scalable solution for delivering genetic instructions to cells. This technology has gained widespread recognition, particularly in the development of mRNA-based vaccines, and continues to drive innovation in fields such as gene therapy, oncology, and protein replacement therapies [1] [2].
However, the successful formulation, characterization, and delivery of mRNA-LNPs present significant analytical and process challenges [3]. Optimizing critical parameters such as particle size, encapsulation efficiency, stability, and purity is essential to ensure efficacy and safety. This application note outlines advanced analytical characterization and quality assessment of mRNA-LNP formulations, using asymmetrical flow field-flow fractionation (AF4) interfaced with multi-detection (MD) system. The MD contains multi-angle static and dynamic light scattering detectors, and two concentration detectors: a differential refractive index (RI) and an ultraviolet (UV) absorption detector.
Results & Discussion
Comprehensive size and shape analyses
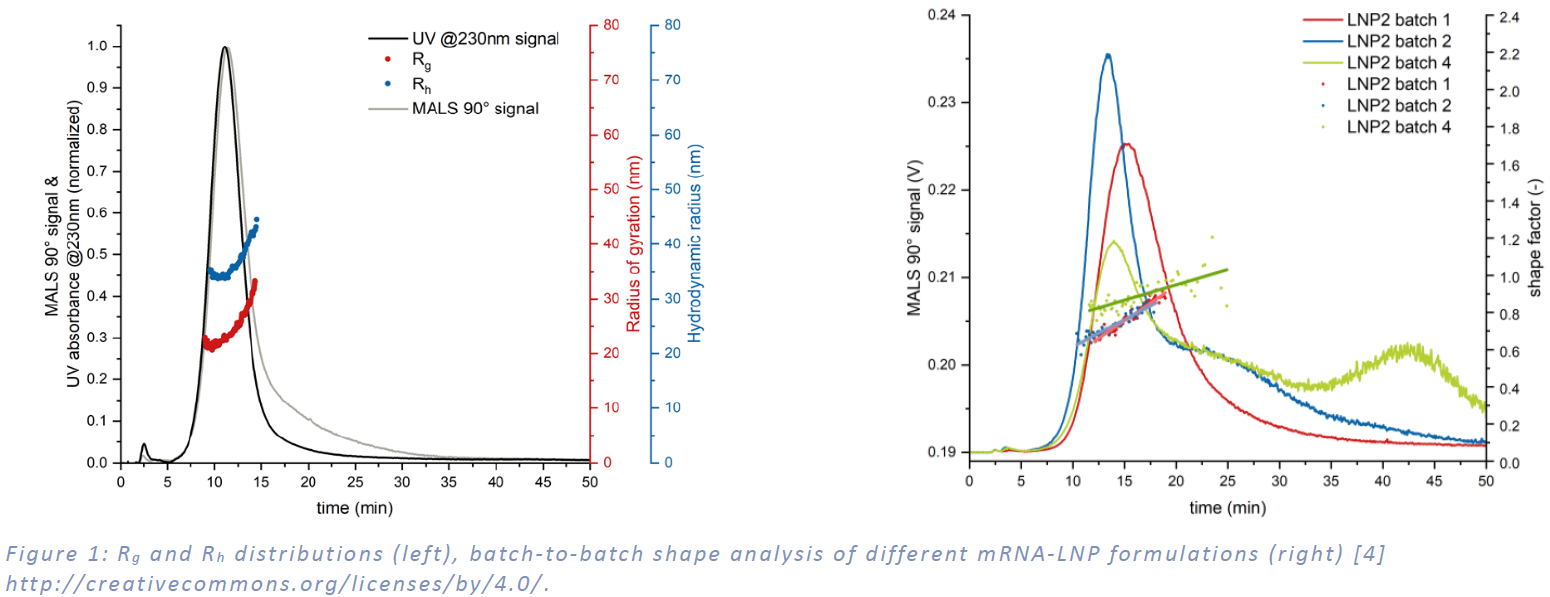
Figure 1 presents a comprehensive analysis of the size and shape characteristics of various mRNA-LNP formulations, as measured by the Postnova MD-AF4 system. Equipped with a MALS and a DLS detector, this system delivers precise, high-resolution particle size distributions derived from root mean square (R.M.S.) radius of gyration and hydrodynamic radii (Rg and Rh), as shown in the left-hand portion of the figure. The sample evaluated in this study displayed a uniform, narrow size distribution, with Rh values ranging from 25 nm to 45 nm and Rg values spanning 20 nm to 30 nm.
The shape characteristics of different LNP batches were assessed by graphing the shape factor (Rg/Rh ratio) against elution time. As shown in figure 1 (right) batches 1 and 2 demonstrated comparable shape factors, suggesting consistent morphology between them. In contrast, batch 4 exhibited a notably higher ratio, indicative of a less dense packed structure or aggregation.
mRNA payload determination
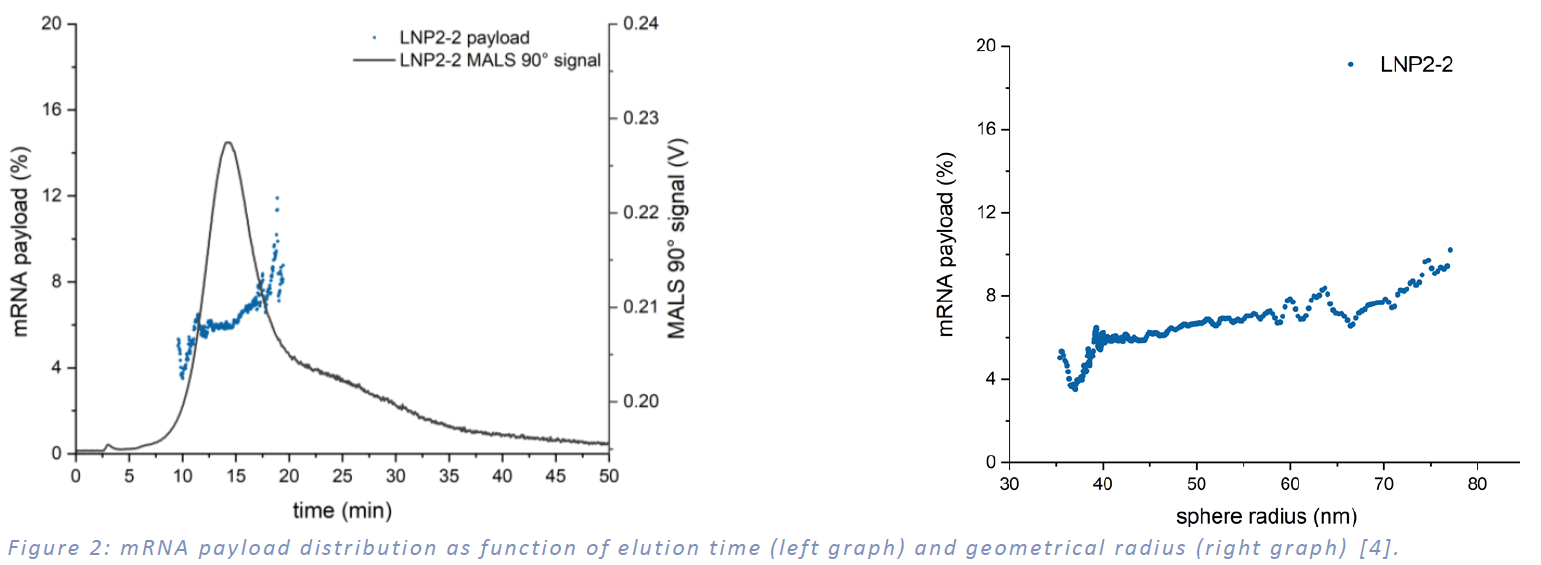
Another key quality control parameter measurable with MD-AF4 is the mRNA payload relative to LNP size. By employing both RI and UV detectors, the mass of mRNA per LNP can be determined. Figure 2 illustrates the distribution of mRNA payload as a function of elution time (left graph) and the geometrical radius of the LNP (right graph). The data reveals that the payload increases with particle size, ranging from 4% to 10%.
Conclusion
The development of mRNA-LNP therapeutics hinges on overcoming complex analytical challenges to ensure consistent quality, efficacy, and safety. The Postnova MD-AF4 system, integrating asymmetrical flow field-flow fractionation with multi-detection capabilities - including UV, MALS, DLS, and RI detectors - offers a robust solution for the comprehensive characterization of mRNA-LNP formulations. As demonstrated in Figure 1, this system provides high-resolution size and shape analyses, revealing uniform particle distributions (Rh: 25-45 nm; Rg: 20-30 nm) and batch-to-batch morphological insights, with Batches 1 and 2 showing consistency, while Batch 4 indicated a distinct dense packed structure. Additionally, Figure 2 highlights the system’s ability to quantify mRNA payload as a function of LNP size, with encapsulation efficiency increasing from 4% to 10% as particle size grows. These findings underscore the MD-AF4 system’s critical role in enabling precise quality control, supporting the optimization, scalability, and reproducibility of mRNA-LNP-based vaccines and therapeutics. For more information on the detailed fractionation conditions and a comparison of MD-AF4 with other analytical techniques for the physicochemical characterization of LNP-RNA therapeutics, e.g., dynamic light scattering (DLS), particle tracking analysis (PTA) or analytical ultracentrifugation (AUC), we recommend further reading in Parot et al., 2024, Journal of Controlled Release [4].
References
[1] J. Lutz, S. Lazzaro, M. Habbeddine, K. E. Schmidt, P. Baumhof, B. L. Mui, Y. K. Tam, T. D. Madden, M. J. Hope, R. Heidenreich, M. Fotin-Mleczek, “Unmodified mRNA in LNPs constitutes a competive technology for prophylactic vaccines,“ Nature, 2017, 29.
[2] Y. Zong, Y. Lin, T.Wei, Q. Cheng, “Lipid nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy,“ Advanced Materials, 2023, 35(51), 2303261.
[3] S. S. Nogueira, E. Samaridou, J. Simon, S. Frank, M. Beck-Broichsitter, A. Mehta, “Analytical techniques for the characterization of nanoparticles for mRNA delivery,“ European Journal of Pharmaceutics and Biopharmaceutics, 2024, Nr. 198, 114235.
[4] J. Parot, D. Mehn, H. Jankevics, N. Markova, M. Carboni, C. Olaisen, A. Hoel, M. Sigfúsdóttir, F. Meier, R. Drexel, G. Vella, B. McDonagh, T. Hansen, H. Bui, G. Klinkenberg, T. Visnes, S. Gioria, P. Urban-Lopez, A. Prina-Mello, S. E. Borgos, F. Caputo, L. Calzolai, “Quality assessment of LNP-RNA therapeutics with orthogonal analytical techniques”, Journal of Controlled Release, 2024, 367, 385-401, https://doi.org/10.1016/j.jconrel.2024.01.037.