Introduction
Lentiviruses are one of the most efficient tools of gene delivery which can deliver significant amounts of genetic information into host cells. As with all drug research, lentivirus vector development requires a thorough characterization of the purified viral product [1]. A variety of analytical tools such as Dynamic Light Scattering, Nanoparticle Tracking Analysis, and Flow Field-Flow Fractionation have been utilized to characterize the vector products in terms of size distribution and particle count [2]. In this application note, Centrifugal Field-Flow Fractionation (CF3) interfaced with a Multi-Angle Light Scattering (MALS) detector was used to characterize a lentivirus product before and after RNA transduction. The CF3-MALS system was previously used to characterize liposomal drug delivery products [3-4]. CF3, a sub-technique of the FFF family of instruments, uses centrifugal force to separate particles based on their buoyant masses. The separation in CF3 takes place in a curved channel with stainless steel walls. The channel is spun in a centrifuge basket to generate a centrifugal field perpendicular to the liquid channel flow.
Experimental Details and Results
The lentivirus samples had titer concentrations of 108 particles/mL and were diluted 2-fold using the CF3 carrier solution (phosphate-buffered saline, PBS) prior to the analysis. The injection volume used was 40 μL. The separation method was a field-decay program with an initial field of 4500 rpm. The channel eluent flow rate was 1 mL/min.
Mass-based Separation by CF3
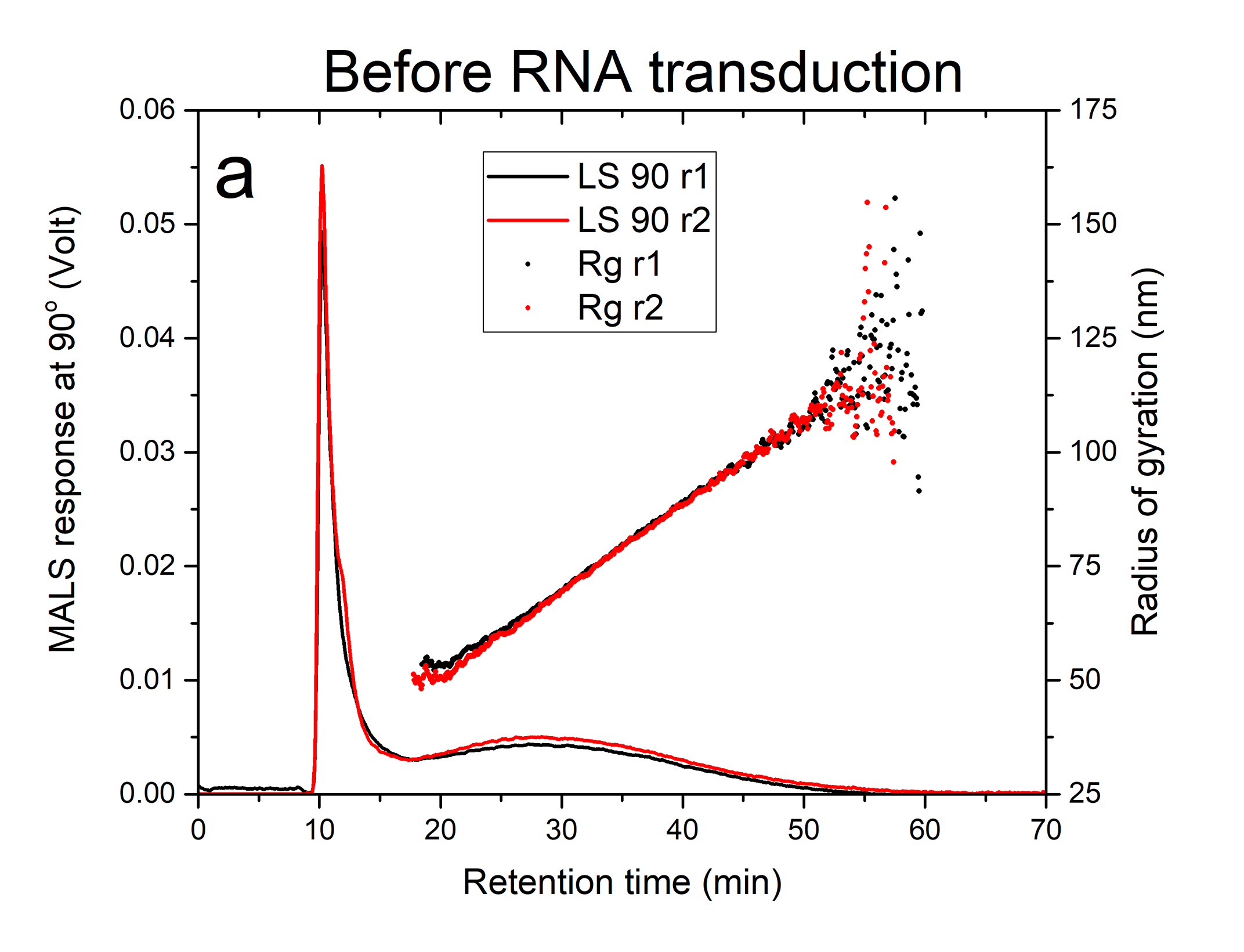
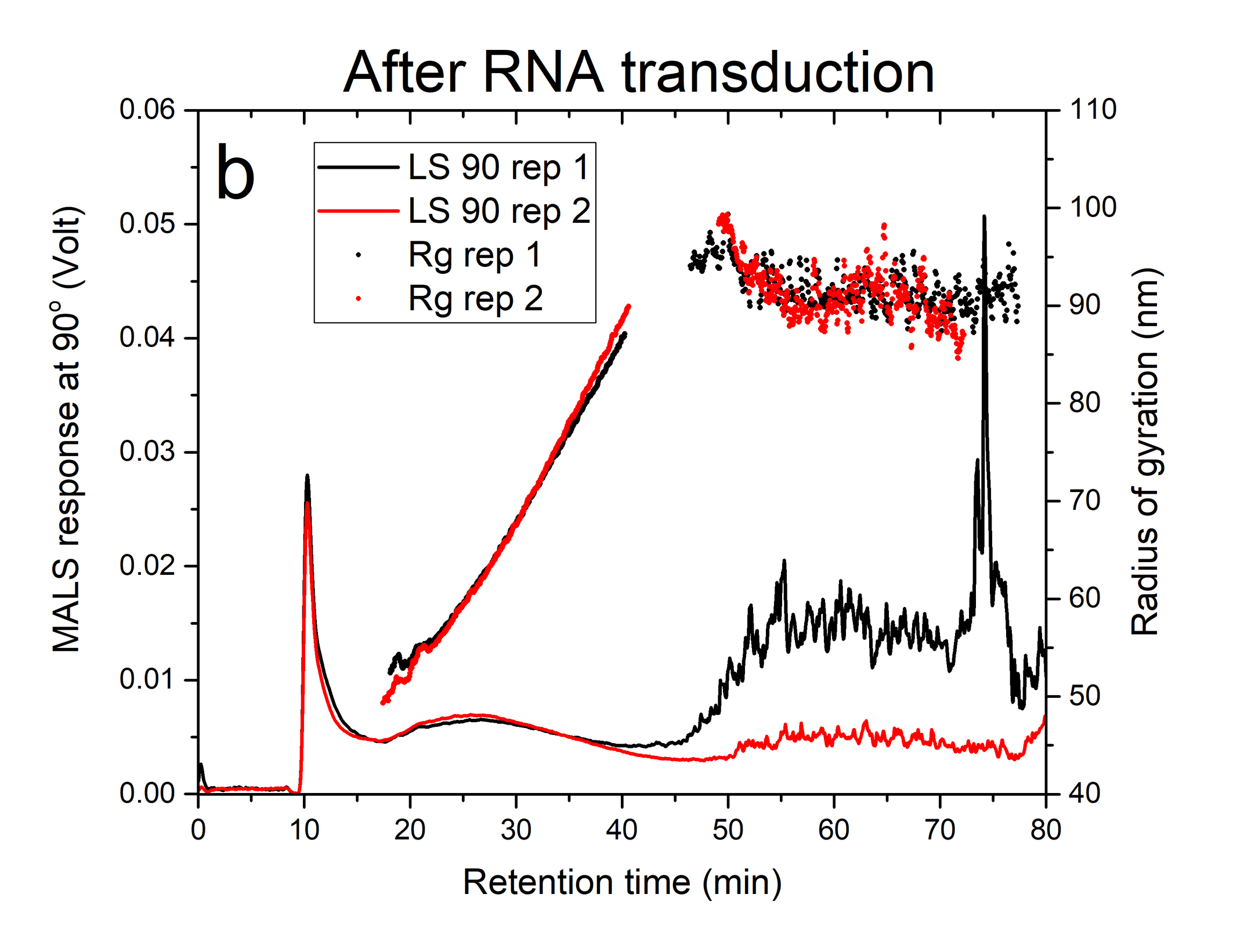
Figures 1a and 1b illustrate the fractograms obtained from the response of the MALS (90° detector angle) for two replicates of the lentivirus sample before and after RNA transduction. The sharp first peak eluting at about 10 minutes of run time is the void peak composed of non-retained and low-mass components present in the sample. The broader peak eluting from 15-50 minutes in figures 1a and 1b represents the lentivirus particle distribution before and after RNA transduction, respectively. There was no shift observed in the retention time when the lentivirus particles were loaded with RNA. This may suggest that the mass of RNA cargo was probably below the minimum detectable mass (60 x 10-18 g or 5% of empty lentivirus mass) under the applied field. The extra peak eluting between 60-90 min, only observed for the sample after RNA transduction (figure 1b), suggests the presence of aggregated lentivirus particles.
Size Analysis by MALS
The plots of solid circles in Figures 1a and 1b are radius of gyration, Rg, measured by MALS for the lentivirus product before and after RNA transduction, respectively. The lentivirus monomeric population exhibited a very similar size range of 48-90 nm with a weighted average value of 69 ± 4 nm and 64 ± 4 nm before and after transduction, respectively (see Table 1). The aggregated lentivirus particles, only present after RNA transduction, have a weighted average radius of 91 nm.
Conclusion
Data presented in this application note demonstrate the feasibility of using the CF3-MALS system as a screening tool to check the quality of lentivirus vector products for impurities such as aggregated virus particles. The system can be also utilized to measure the mass of cargo per virus particle if the loading mass is above 60 x 10-18 g or 60 attograms, respectively.
References
[1] A.S. Moreira, C.D. Cavaco, T.O. Faria, P.M. Alves, M.J.T. Carrondo, C. Peixoto, Biotechnology Journal, 2021, 16, 2000019.
[2] E. Hansen, K. Lee, S. Melotti, J. Milling, M. Miatkowski, J. Warren, M. Segura, Vector and Cell Engineering/Manufacturing II, 2016, 24(1), S280.
[3] Centrifugal Field-Flow Fractionation Hyphenated with Dynamic Light Scattering for Size Characterization of Nanoparticle Systems for Biopharmaceutical Applications.
https://www.postnova.com/knowledge/application-notes/application-note/267-id0038.html
[4] Measuring Mass of Liposome Cargo Using Centrifugal Field-Flow Fractionation.
https://www.postnova.com/knowledge/application-notes/application-note/260-id0067.html