Introduction
Adeno-associated viruses (AAV) are small single-stranded DNA-containing protein capsids with an average diameter of 25 nm. AAVs have emerged as leading candidates for gene therapy [1,2]. One of the critical quality attributes that needs to be quantified for a safe and effective AAV product is its stability. In this application note, we describe the application of Asymmetrical Flow Field-Flow Fractionation (AF4) coupled to a multi angle light scattering (MALS) detector and a UV/Vis diode array detector to study the aggregation of two AAV serotypes when they are stressed by heat.
Experimental Details
Two AAV serotypes, AAV1 and AAV5, with stock concentrations of 2x1013 particles/mL, were used for this study. The samples were diluted 5x with the formulation buffer (1x PBS buffer and 0.001% (v/v) 10% Pluronic F-68) prior to analysis. The samples were stressed at 60°C and 75°C for 35 minutes. An AF4 system (Postnova AF2000 Multifl ow) system interfaced with a MALS detector (Postnova PN3621) and a diode array UV-DAD detector (Postnova PN3241) were utilized to analyze the AAV samples using the formulation buffer as the AF4 carrier solution. For each analytical run a 50 L injection of the prepared sample solutions was used.
Separation Results
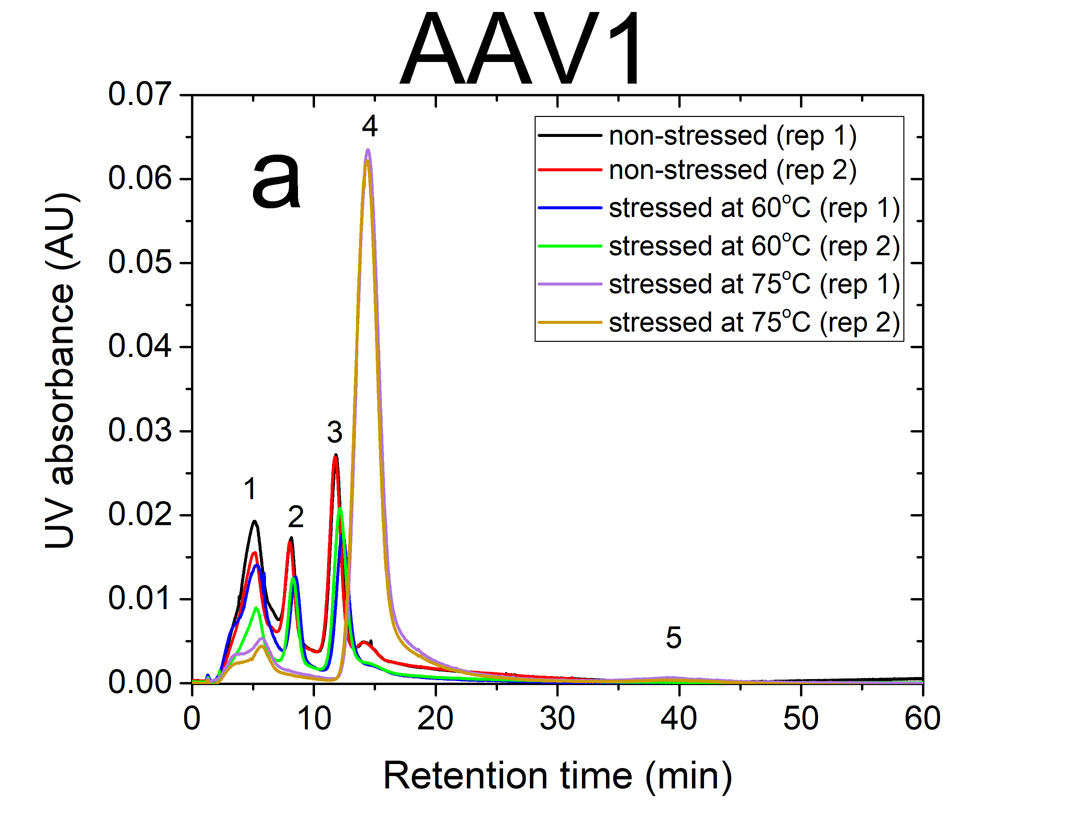
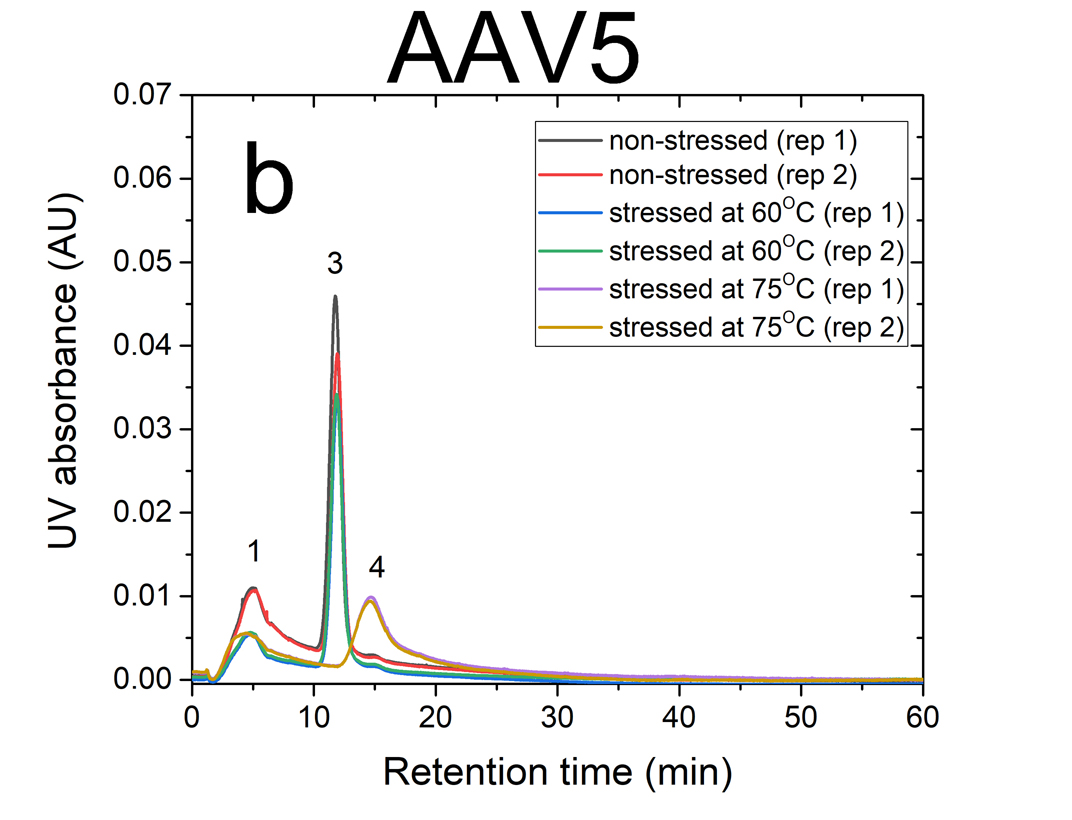
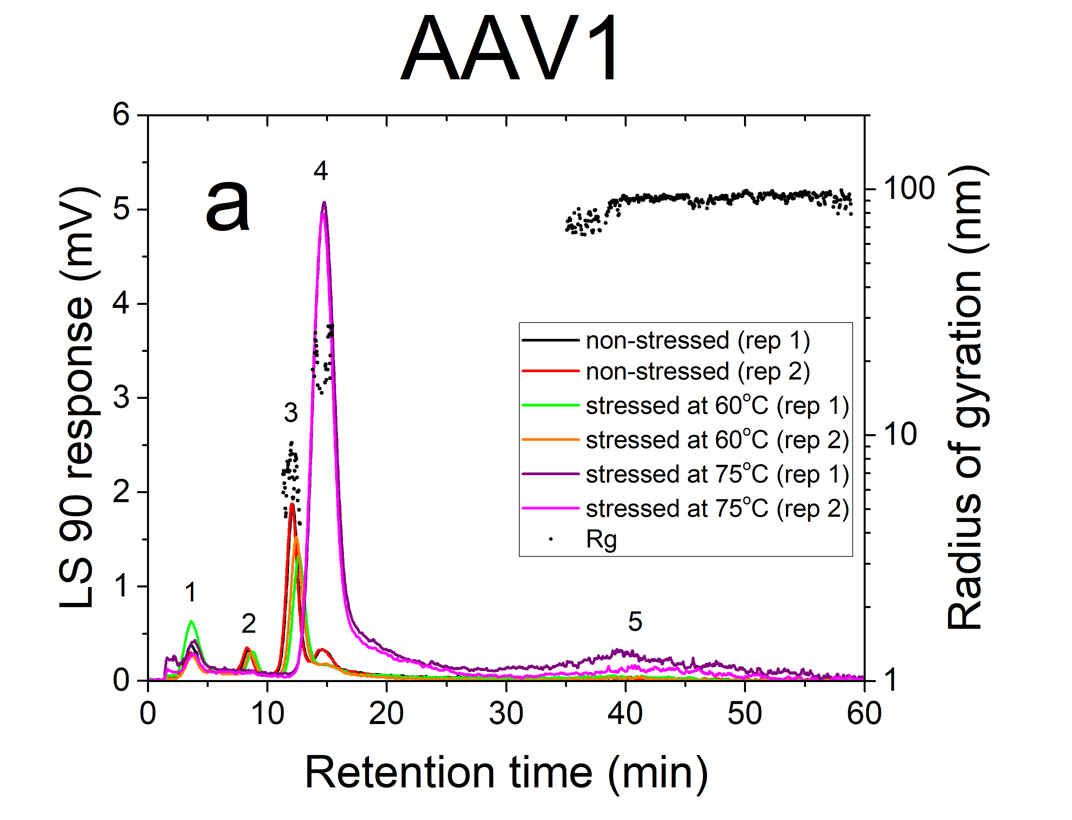
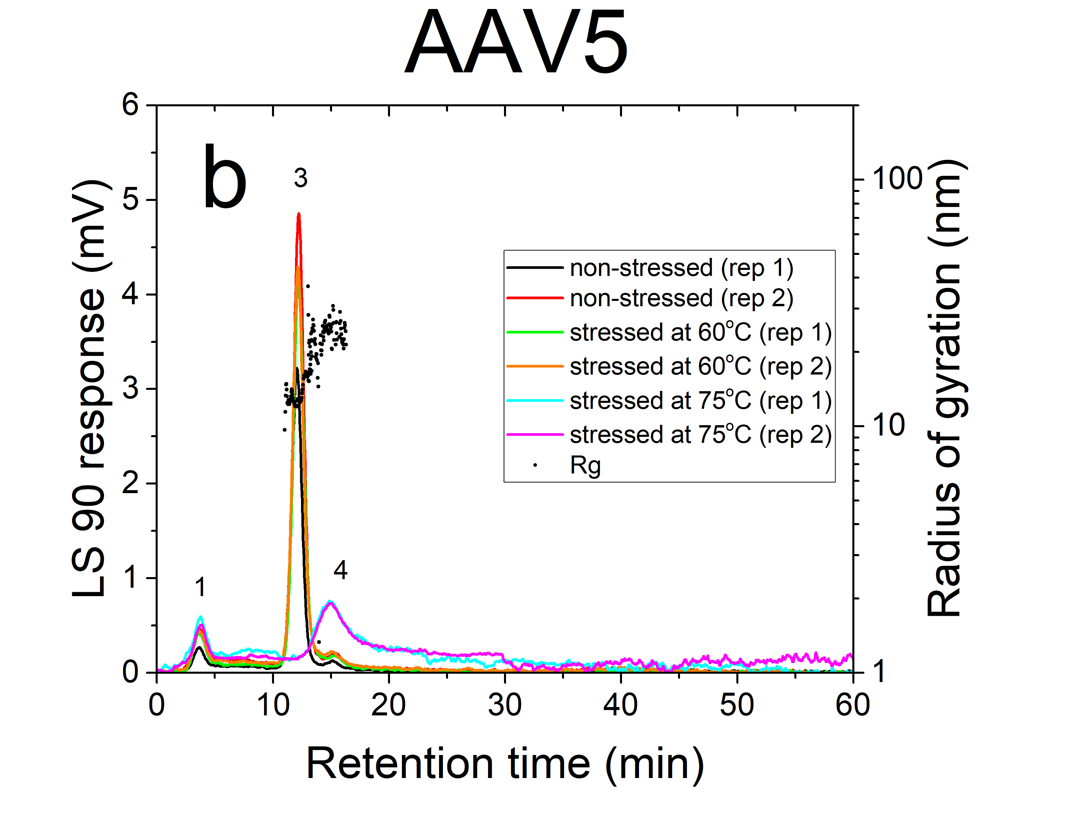
AF4 is a well-suited separation technique to study the aggregation of AAVs. Due to its open-channel architecture, AF4 can separate aggregates much larger than what can be accomplished by column chromatography. Figures 1a and 1b show plots of UV signal obtained at 219 nm absorbance wavelength versus retention time for non-stressed and stressed AAV1 and AAV5 serotypes. The numbered peaks in the graphs are the subpopulations present in the samples which have been separated by AF4 based on their hydrodynamic sizes.
Eluting first are the smaller viral fragments in peaks 1 and 2 followed by the virus monomer and oligomers in peaks 3 and 4 respectively. Peak 5 contains the larger virus aggregates that elute latest in the separation (35 to 60 minutes), which are detected only for the stressed AAV1 sample at 75°C. For non-stressed AAV1, monomeric and oligomeric viral particles constitute about 26% and 7% of the sample, respectively. The non-stressed AAV5 sample contained more monomer than AAV1, with 43% in the monomeric and 5% in the oligomeric forms.
The data indicates that both serotypes are reasonably stable up to 60°C. However, they exhibited a signifi cant change in their size distributions when they were stressed at 75°C. In both stressed samples, the monomer (peak 3) vanished completely, the oligomer peak (peak 4) grew substantially (66% for AAV1 and 26% for AAV5). This suggests that the virus particles form oligomers as they undergo heat stress.
Size Measurement
The AAVs’ radius of gyration (Rg) in non-stressed and stressed samples was obtained from evaluation of the MALS signal intensities at different angles. The Rg data points (black circles) of non-stressed and stressed AAV1 and AAV5 samples are shown in fi gures a and 2b, respectively. Each point represents a size measurement for a volume of sample passing through the MALS fl ow cell. On average, virus monomers (peak 3) are in the order of 8-10 nm in radius. The oligomer peak varies from 10-30 nm in radius. The large aggregates (peak 5), only present in the stressed AAV1 sample, are ~100 nm in radius and represent approximately 2% of the population.
Conclusion
Both AAV1 and AAV5 were stable in the formulation buffer below 60°C. The data suggest the heat stress induces oligomerization when the samples are heated at 75°C. AF4 coupled to MALS and UV detectors can provide valuable information to study aggregation of viruses and virus-like particles when they are exposed to heat stress.
References
[1] E. Hastie & R.J. Samulski, 2015, Human Gene Therapy, 26(5), 257–265.
[2] M.F. Naso, B. Tomkowicz, W.L. Perry III & W.R. Strohl, 2017, BioDrugs, 31, 317–334.