University of Utah, Salt Lake City, UT, USA
Introduction
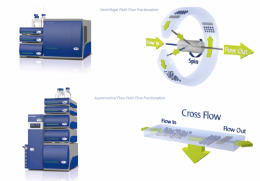
Figure 1: Separation mechanism diagrams for CF3 and AF4.
Exosomes are small extracellular vesicles containing nucleic acid and proteins with a great potential for cancer diagnostics1,2 and therapeutic applications. Characterization of exosomes is challenging due to their inherent heterogeneity and complexity. Multiple fractionation types may be necessary to provide a variety of measurands. Asymmetrical Flow Field-Flow Fractionation (AF4) and Centrifugal Field-Flow Fractionation (CF3) are high resolution separation techniques for fractionation of macromolecules and biological nanoparticles based on their hydrodynamic size (AF4) and mass (CF3). In this study the goal was to characterize the MCF-7 tumor exosome sample by size using AF4 coupled to multi angle light scattering (MALS), and by mass using CF3. The density of exosomes in CF3 fractions was obtained using hydrodynamic diameter and buoyant mass measured by Nano Tracking Analysis (NTA) and CF3 respectively. Fractions were collected along both size and mass distributions and analyzed by polymerase chain reaction (PCR) for enrichment of the micro-RNA21 (miR21) cancer biomarker.
The CF3 channel encircles the rotational axis and spins to generate a centrifugal field perpendicular to the channel flow. Particles with lower mass can diffuse against the centrifugal field into faster channel flow profiles, and are eluted earlier than more massive particles. In AF4, the separation field is generated by a cross flow perpendicular to the channel flow. Particles with smaller size can diffuse against the cross flow field into faster channel flow profiles, and are eluted earlier than larger particles.
Experimental Details and Results
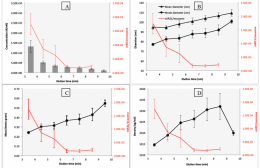
Figure 2: Plots of FFF elution time (x-axes) vs miR21 enrichment/exosome (right y-axes). The left y-axes vary by plot and include exosome concentration (A), diameter (B), mass (C), and density (D).
The relationship of miR21 enrichment to physicochemical parameters measured by AF4 and CF3 is shown in Figure 2. From Figure 2(B) it appears that exosomes enriched with miR21 tend to be smaller in size as measured by AF4-MALS with NTA, as the enrichment (right y-axis) decreases as size (left y-axis) increases. Figure 2(C) suggests a decrease in miR21 enrichment with increased exosome mass as well, as measured by CF3 separation. Combining mass separation with fraction measurements by NTA yielded measurements of exosome density; this is plotted vs. miR21 enrichment in Figure 2(D). The enrichment decreases with increasing density.
Conclusion
The MCF-7 tumor exosome sample exhibited a broad size distribution ranging from 30 to 100 nm in R.M.S radius (MALS data, not shown) and 50 to 200 nm in hydrodynamic diameter. The heterogenous nature of these samples makes batch dynamic light scattering or NTA measurements prone to error, and highlights the usefulness of FFF for separation and characterization of these complex biologic samples. The study showed that the enrichment of miR21 cancer biomarker was highest in the tumor exosomes with the smallest size, smallest mass, and lowest density.
References
[1] Peng et al. Oncotarget. 2017 Jul 4; 8(27): 44893-44909.
[2] Wei et al. Chin J Cancer. 2011 Jun; 30(6): 407-414.